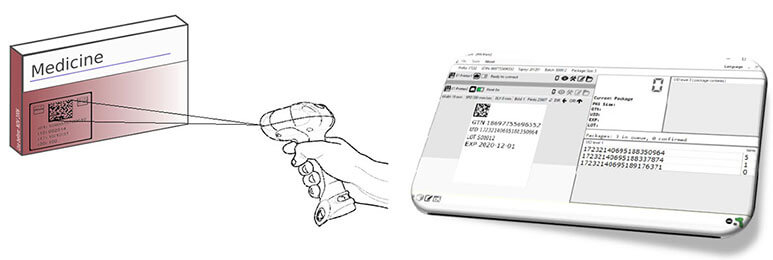
Prevent Recalls Arising from Packaging & Labelling
When products escape the labelling and packaging component of a manufacturer’s production system, recalls happen! In fact, in a recent FDA presentation, the causes for some recall events (and their percentage of total recalls) were identified as follows:
- Incorrect/missing production lot number – 6%
- Label mix-up – 5%
- Mis-carton/mis-packaging – 5%
A New & Superior Way of Coding and Marking Drug Products

Pharmaceutical track & trace systems play an important role in diminishing the occurrence of these non-conformances. And, Printing Verification and Control Devices can be used:
- to assure imprinting conforms to the batch record
- for drug product, case and carton labels
A robust packaging and labeling system:
- Prevents labeling mix-ups
- Provides traceability information for the lot
- Improves visibility of key information
- Ensure legibility of drug name or strength on blister packaging
- Offers long lasting ink
- Can be used to bring attention to important product information on shipping cartons.
For a guide to provide direction to sponsors, manufacturers and license holders in designing safe and clear labels and packages, see Government of Canada’s Good Label and Package Practices Guide for Prescription Drugs. With RN Mark printers, you can quickly and accurately code your products at all levels. From marking the product itself all the way to final packaging.